Introduction
In Part II we explained how in the most simple terms that Leptin and Insulin appear to oppose each other since high sugar intake from processed food and fast food maintain continuous high insulin levels blocking the action of Leptin that is supposed to signal satiety. However, both Leptin and Insulin are hormones that are supposed to signal the hypothalamus to decrease eating as well as other neurotransmitters and hormones listed below:
Hormones/Neurotransmitters involved with ANOREXIGENIC Function – STOP EATING
| Neurotransmitter/Hormone | Source |
| alpha-Melanocyte-stimulating hormone (a-MSH) | POMC neuron in the hypothalamus |
| Leptin | Adipose tissue ( Fat cells) |
| Serotonin | The GI Tract |
| Norepinephrine | Adrenal gland |
| Corticotropin-releasing hormone (CRH) | The PVN of the hypothalamus |
| Insulin | Beta cells of the pancreas |
| Cholecystokinin (CCK) | Duodenum cells |
| Glucagon-like peptide (GLP) | Solitary Tract of the brainstem |
| Cocaine- and amphetamine-regulated transcript (CART) | Neuron in the arcuate nucleus, Hypothalamus |
| Peptide YY (PYY) | The small/large intestine |
| Enterostatin | Small intestine |
| Oxyntomodulin | L cells in ileum & colon |
So you can appreciate that it’s not just leptin/insulin interaction but it is more complicated as indicated by the number of neurotransmitters/hormones that activate upon food consumption to regulate intake and activate a decrease in eating. So what dysfunctions to cause obesity ??. Let us investigate a little further. Ultimately the central nervous system is central to the multiple signalling that takes place when food is consumed, so let us use the diagram below and try to understand these signalling pathways and their interaction.
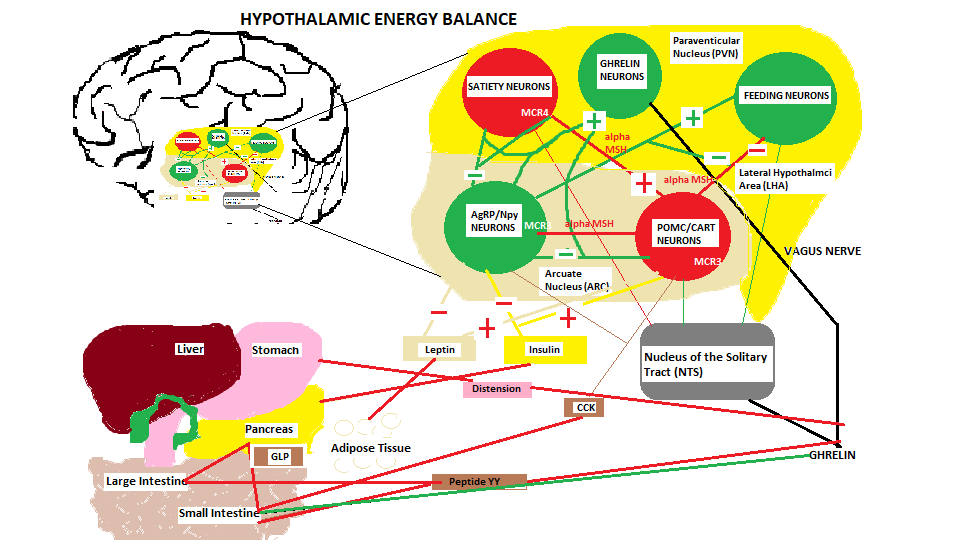
Energy Balance regulation to inhibit food consumption toward satiety
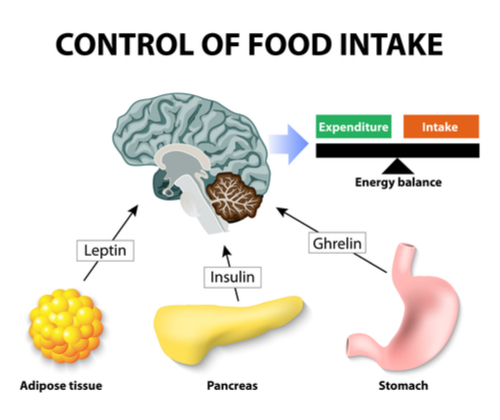
When food begins entering the GI tract volume information within the stomach is sensed by mechanical distention that signals the hypothalamus, and at the same time the hormones and neurotransmitters start to send on-going meal signals which are also referred to as satiation signals, or within-meal intake inhibitory signals, to begin regulation of food intake. These satiation signals are derived from various sources as described in the list above and shown in the diagram above.
Alpha-Melanocyte-stimulating hormone (a-MSH)
Peptide hormones that are secreted by the Proopiomelanocortin (POMC) neuron in response to a leptin signal and used to suppress appetite. The same substance is secreted by the melanocytes in the skin in response to UV sunlight increasing the synthesis of melanin protecting the skin from DNA damage and skin cancer ( take heed all dermatologists ). The pituitary gland releases adrenocorticotropic hormone which is essentially the same as alpha-MSH to stimulate the adrenals to release cortisol.
Leptin
Referred to as the ‘hormone of energy expenditure’ that is secreted by adipose tissue which is part of this energy balance regulatory system that monitors fat tissue depots and signals to the hypothalamus based on fat mass ( into neuron receptors on the POMC/CART neuron synapse) to inhibit hunger. This hormone is driven in accordance with fat mass exponentially and is higher between midnight and early morning which is to be expected, interacting with Melatonin to suppress appetite throughout the night and early morning. 72 hr fast decreases leptin levels, as well as exercise, and increased by emotional stress and the presence of insulin and obesity as would be expected.
Serotonin
We have discussed this neurotransmitter in detail in previous articles debunking medical myths that stated low serotonin levels cause depression, but in this case it is part of the appetite suppression network within the hypothalamus. This is very interesting and almost ironic because it appears that tryptophan, the precursor of serotonin can only enter the brain after consuming something sweet or starchy carbohydrate. Although tryptophan is an amino acid, it is in competition with other protein derived amino acids in terms of crossing the blood brain barrier (BBB) when protein is consumed. Eating sweet or starchy carbohydrates, not fruit, and once digested to glucose, it enters the bloodstream triggering an insulin release causing amino acids and other nutrients to enter various cells of the body, but tryptophan stays behind in the blood but at this point it is able to cross the BBB where it is quickly converted to serotonin. This effect most people have experienced while waiting for a meal to be served and you decide to eat some bread because you feel hungry, but half an hour into the meal you might find your appetite has been slightly suppressed. This explains why some individuals have sugar cravings because of low serotonin levels ( ….so now I understand the Serotonin and depression association, they are missing a bar of chocolate….lol).
Norepinephrine
This monoamine neurotransmitter activates 2 alpha adrenoceptors located on the anorexigenic (alpha 1: stop eating) and the orexigenic ( alpha 2: appetite stimulus) neurons located in the hypothalamic Paraventricular nucleus ( PVN) shown on the diagram. This activation is under the control of our circadian rhythm cycles where alpha 1 is activated during the dark cycle to suppress eating, and alpha 2 is activated during the day.
Corticotropin-releasing hormone (CRH)
Since this hormone is released by the hypothalamus to stimulate the pituitary gland to release adrenocorticotropic hormone, during a stress event, it is this hormone that also affects appetite as one has probably experienced when under stress you lose appetite. Having said that stress can not only suppress appetite and also stimulate it, and I am sure everybody has met a person that has driven themselves to be overweight because of depression, driven by high stress and physiologically and psychologically they have compensated for it by a kind of reward for the tribulation they are going through. I think initially when stress begins to kick in there is no room to eat because you have no appetite, but as time progresses the adrenals start to become fatigued and the body begins to adapt to the high stress and this sense of reward begins so the tables are reversed and you start to eat possibly non nutritional comfort food.
CRH is released from the hypothalamus which triggers adrenocorticotropic hormone ( ACTH) to be released from the pituitary which in turn stimulate the adrenals to release Glucocorticoids (Cortisol) which enhances glucose availability through protein breakdown and ultimately energy mobilization to meet the perceived threat. However, this energy mobilization also alters food intake since the adrenal also releases corticosterone, another hormone that has been shown to increase the need to consume sucrose. Three studies conducted in the 90’s uncovered a link between HPA activity and abdominal obesity. In 2009 a double blind study ( name: CRH-stimulated cortisol release and food intake in healthy, non-obese adults ) was conducted using 8 female and 6 male subjects who were dosed with CRH at a level that would not elicit a stress response and then their food intake was measured. Their findings :
‘ Low dose CRH administration significantly increased food intake compared to a placebo injection in healthy, non-obese adults, as measured by both calories and total grams consumed. The magnitude of the peak cortisol response to CRH was a strong predictor of subsequent food intake. These data extend growing evidence of a link between stress response systems and human eating behavior, by suggesting that activity within the HPA axis – our central, neuroendocrine stress response system – is neurobiologically linked to food consumption.’
Insulin
A Hormone secreted by the pancreas when triggered by glucose in the bloodstream. After any food that is transformed into glucose such as carbohydrates, starch ( rice potatoes) and excessive protein triggers insulin, and its purpose is to message the bodies cells to uptake the glucose and feed the cells. If this occurs as normal then insulin works as an appetite suppressant once the cells have been fed.
Cholecystokinin (CCK)
This is a peptide hormone secreted by enteroendocrine ( I ) cells in the duodenum and Jejunum stimulating the release of digestive enzymes from the pancreas and bile from the gallbladder for fat and protein digestion and also acts as a hunger suppressant.
Glucagon-like peptide (GLP)
Another peptide hormone known as an ‘incretin’ or metabolic hormone secreted by L Cells in the ileum and colon and released in response to food intake. It stimulates insulin secretion (incretin hormone) from the Pancreas when there is glucose in the bloodstream ( note: the mad mad world of pharmaceuticals appear to have at least 6 GLP-1 receptor agonist, exendin-4 type drugs to treat diabetes II because it mimics what the pancreas does, but I am not sure why they would want to do this since Diabetes II is a problem of cellular insulin resistance not a problem with insulin secretion since in Diabetes II there is too much circulating insulin not too little). This hormone also inhibits glucagon secretion ( pancreatic glucagon secretion occurs when blood glucose falls too low), inhibits gastric motility ( delaying stomach emptying slowing down insulin secretory spikes) and secretion acts as an ‘ileal brake’, thus stimulating a satiety effect, and appetite suppressant.
Cocaine- and amphetamine-regulated transcript (CART)
CART is a neuropeptide that is released by the same POMC neuron that produces alpha-MSN. It is involved with reward, feeding and stress and acts like an endogenous psychostimulant producing similar behaviour as cocaine and amphetamine ( so why would you want to these outside of the body when the body produces the same effects for free..lol). From our diagram you can see satiety notification inputs (+) from insulin and leptin. The other (-) inputs from Leptin and Insulin produce a negative signal to the regulatory feeding neuron notifying this neuron that satiety has not been reached and to continue feeding.
Peptide YY (PYY)
This is a peptide hormone secreted by enteroendocrine ( L ) cells in the Ileum and Colon whose mechanism of action after a meal inhibits gastric motility ( metabolic energy driven movement), increases water and electrolyte absorption in the colon, and reduces appetite. These same cells also secrete Oxyntomodulin, a peptide hormones also used to suppress appetite. This peptide is also raised when dietary fibers from fruit, vegetables and whole grains are consumed increasing the speed of transit of intestinal chyme into the ileum to induce satiety.
Enterostatin
This is a derived peptide ( amino acid chain ) hormone that is produced by a protein co-enzyme procolipase that is secreted by the pancreas into the GI Tract which is activated by trypsin and used to control fat intake. Its effect is to lower insulin levels, provides stimulatory sympathetic signals to brown adipose tissue for thermoregulation ( body warmth ), and adrenal corticosteroid secretion to regulate carbohydrate intake, as a result it is designed to initiate a sense of fullness in the stomach.
Oxyntomodulin (OXM)
This is another incretin metabolic hormone that is released from L cells in the ileum and colon in response to food intake that stimulate a decrease in blood glucose levels by stimulating an increase in insulin release from the pancreas. This action also reduces gastric emptying, reduces gastric acid secretion and reduces food consumption. Oxm works together with GLP 1 and binds the GLP 1 receptor expressed in the Nucleus Solitary Tract ( NTS) in the brainstem and Arcuate Nucleus (ARC) of the hypothalamus. It is supposed to promote weight loss by suppressing ghrelin and leptin.
Mechanism of Action ANOREXIGENIC Function – STOP EATING
Introduction
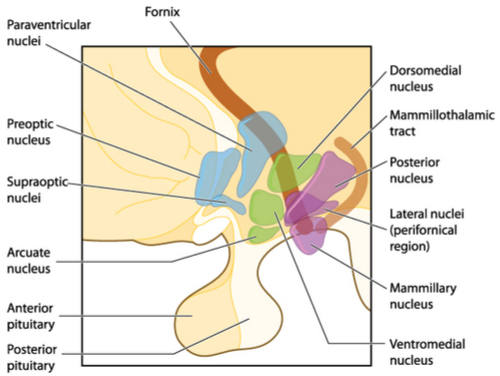
The 2 main centres in the hypothalamus that regulate feeding and satiety are the Lateral Nucleus and the Ventromedial nucleus respectively. The diagram below shows the locations of these areas. For this section we will concentrate our discussion on satiety and in the next article we will focus on feeding.
Consuming food and its regulation is divided into 2 parts, short term and long term which is absolutely necessary. If short term control were absent, the host would continue to eat because digestion takes hours (long term scenario) and if only regulatory feedback occurred after digestion the host would experience great discomfort because of uncontrolled food consumption..wait a minute is this what is happening in obesity ?. Short term eating creates the necessary satiety signals to control eating, and once digestion is complete the host will eventually experience hunger and the cycle begins again.
Satiety ( Anorexigenic )
(Refer to diagram ‘hypothalamic energy balance’ above)
The satiety control centre, the Ventromedial nucleus (VMN) has several pathways and projections to/from the lateral hypothalamus (LHA) and the arcuate nucleus where hypothalamic neuron Proopiomelanocortin (POMC/CART) that processes insulin and leptin satiety signals. This neuron also produces a-MSH an appetite suppressing hormone that is sent to other satiety neurons located in the Paraventricular nucleus (PVN). Upon the progression of the food consumption other satiety signals are initiated which include the Serotonin precursor tryptophan that enters the hypothalamus via the blood brain barrier, norepinephrine that enters via the bloodstream to an alpha 1 receptor located on the satiety neurons in the PVN, A CRH signal released within the hypothalamus to activate the HPA axis and the secretion of corticosterone from the adrenal gland, CCK secreted by the I cells located in the duodenum and jejunum is a hormone used to stimulate bile and pancreatic enzyme release for fat/protein digestion. Another satiety signal called Glucagon like Peptide (GLP) is released by the L cells located in both intestines upon sensing the presence of food in the intestines. Since this is an ‘incretin’ hormone it stimulates the pancreas to secrete insulin. The L cells also secrete another hormone called Peptide YY (PYY) upon food intake rising to peak levels in the bloodstream 1-2 hrs after the ingesting of the meal, especially a meal with a high fat content. Another incretin hormone Oxyntomodulin (OXM) is produced by the L cells which also stimulates the pancreas to release insulin and works in conjunction with GLP, but how they work together is unknown. Enterostatin, another hormone that is released by the pancreas is responsible for providing a sense of fullness in the stomach of the host.
Conclusions
In this article we have discussed the satiety side of the hypothalamic energy balance system and the various hormones and neurotransmitters involved with regulating food consumption. In the next article we will discuss the other side of the ‘equation’ the feeding component of this enormously complex regulatory system that science still does not fully understand and we will explore how obesity occurs when so much control and regulation is in place. In addition we will discuss the crucial role of the central nervous system and the Nucleus of the Solitary Tract (NTS) and its afferent neuronal connection to the vagus nerve.
References/Acknowledgments :
- The physiology of glucagon-like peptide 1 Holst 2007 NCBI
- Mechanisms of Action of GLP-1 in the Pancreas Doyle & Egan 2007 NCBI
- Incretin hormone Diabetes self management 2013
- Serotonin: What it is and why it’s important for weight loss Judith Wurtman 2010 Psychology Today
- Norepinephrine and the control of food intake Wellman 2000 NCBI
- melanocyte-stimulating hormones, incretins, Wikipedia
- CRH-stimulated cortisol release and food intake in healthy, non-obese adults Sophie George et al 2009 NCBI
- Textbook of physiology
- Regulation of Energy Balance and Body Weight by the Brain: A Distributed System Prone to Disruption Lucy Faulconbridge & Matthew Hayes 2011 NCBI
Author: Eric Malouin