Featured foods : Sunflower seeds, Quinoa, Oatmeal
Introduction
The focus in this article is on the nutrients required for mitochondrial function. To understand how important, and also what they are, we include a review of the energy that is derived within each of our cells. This is crucial to life itself, since without energy production we would be unable to move but above all we could not exist. Thus energy is manufactured within the mitochondria apparatus that exists in all cells of the body, the amount of which are very low in skin cells but can number in their thousands for muscle cells, since they are required to move all of the time. Energy production is a consequence of a complex process called the Electron Transport chain ( ETC) which we will describe in relative detail and how dietary nutrients fit into the picture. I have also included some seeds and grain charts that contain some of the vital nutrients required for ETC function.
Cellular structure and the Electron transport chain
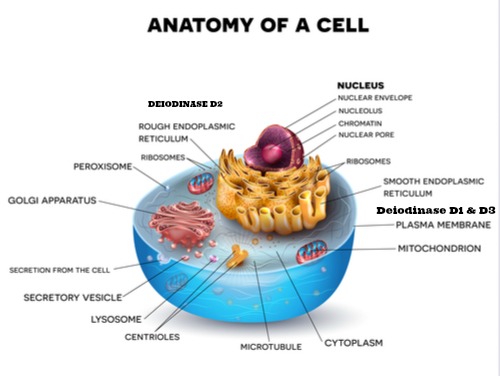
We have seen this diagram before showing the constituent parts of a mammalian cell, but for this discussion we want to home in on the mitochondrion. Two are shown in the diagram but in some active parts of the body requiring movement like muscle especially the heart there are literally thousands of mitochondria within each cell and more can be created depending on the demand of the host such as during intense exercise. Within each mitochondria there are thousands of individual electron transport chains as well. So it is hard to imagine the sheer complexity of an individual cell microscopic in nature. The next diagram shows the mitochondria up close and a closer look at the energy factories within the mitochondria called the Electron transport chain (ETC).
How we generate cellular energy
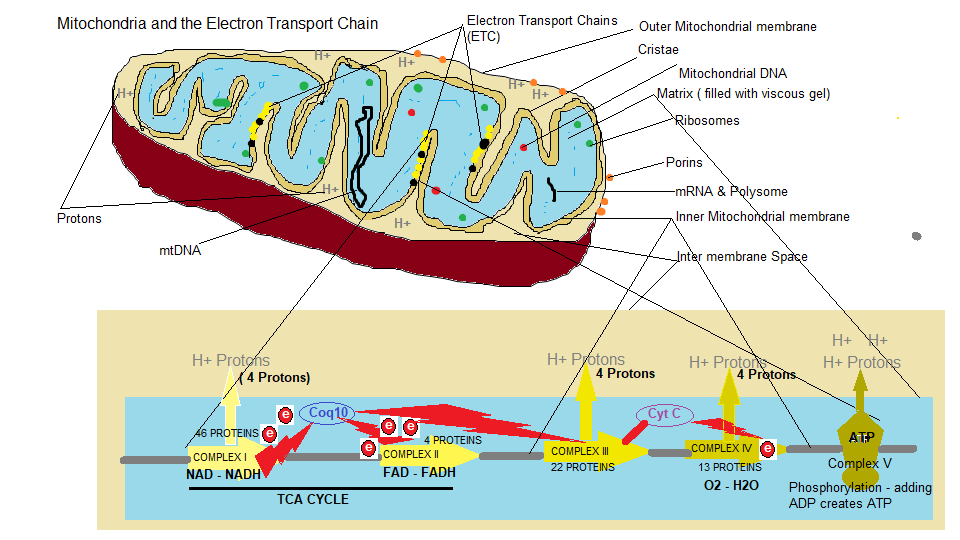
Generating Cellular energy is also known as ‘Oxidative metabolism’. Basically, the enzymes involved in the TCA cycle and the ETC combine food particles from our diet with oxygen to create a proton pump mechanism creating energy. This energy mechanism begins in the Cytosol which is the intracellular fluid inside the cell compartmentalised by internal membranes and part of the cytoplasm shown in Diagram 1 ‘Anatomy of a cell’. Within the cytosol a process called Glycolysis takes place. Glycolysis involves the conversion of glucose into Pyruvic acid or what is referred to as Pyruvate. This acid can be converted back into glucose through a process called gluconeogenesis that the body uses to maintain blood glucose levels. The glucose to pyruvate conversion involves a 10 step chemical process where 1 molecule of glucose can produce 2 molecules of pyruvate. The pyruvate is then transported to the mitochondrial matrix where an enzyme complex called Pyruvate Dehydrogenase links glycolysis to the TCA cycle ( also known as the Krebs cycle or citric acid cycle which is shown above at the bottom of the duagram ‘How we generate cellular energy). The pyruvate dehydrogenase complex is composed of 3 enzymes and 5 cofactor complex which oxidises pyruvate to produce acetyl-coA; a group of coenzymes which breakdown the pyruvate that transfer electrons to NAD+ to produce NADH.
NAD – NADH, FAD – FADH
We came across NAD ( Nicotinamide Adenine Dinucleotide ) coenzyme when we discussed the 90 essential nutrients and energy production. NAD is an oxidised agent, that is an electron acceptor, and once an electron is donated it becomes NADH the reduced version converting into an electron donor. As shown in diagram 2 ‘Mitochondria and the Electron Transport Chain’ NADH donates 2 electrons to Coq10 ( which is actually Ubiquinol the reduced version of Ubiquinone or Coenzyme Q10) a lipid soluble carrier. The overall reason for the ETC, is to create enough protons (Hydrogen atoms) from the proton pumps ‘Complex I, III, IV and V ( ATP synthase)’ to generate sufficient energy to drive the cell. So the first energy molecule is NADH (Complex I), and the second is FADH (Flavin Adenine Dinucleotide the reduced version of FAD) (Complex II). which from, our previous discussions in the articles the 90 essential nutrients, is the Fat burner ( electron donation by fatty acid metabolism). FAD is used in the ETC as an input channel to burn fatty acids. By pumping protons from the matrix to the intermembrane space creates a high concentration of protons between the membranes and a low concentration in the matrix, thus creating a electrochemical gradient for stored energy. At the end of an energy cycle FADH and NADH are changed back to their oxidised forms, and then FAD and NAD are ready to be used in the next cycle of glycolysis to keep the energy process running.
Cytochrome C and Complex IV ( Cytochrome C oxidase)
Cytochrome C is a heme protein ( needs iron) that uses oxygen to move electrons, and also splitting and binding to a hydrogen proton ( H+) to form water H2O. It is pertinent to point out here that the electrons produced by NADH and FADH go from a high energy state to a low energy state into water which results in a release of energy. The question is why have this stage at all, why not just donate electrons directly from the TCA cycle ( NADH + FADH) ?. The simple answer is that there would be a an excessive energy drop where the reaction would release energy as heat, so complex IV acts as a buffer to slow down electron transfer using oxygen as a transport to accomodate energy storage rather than released as heat. I suppose the other question is, why have an electron transport chain (ETC) at all, since the process of glycolysis and the conversion of glucose to pyruvate can also generate energy. Well, the ETC can be considered as an energy amplifier, since glycolysis can only harness less than 10% of the energy produced from the glucose, which amounts to only 2 molecules of ATP/1 glucose molecule. Whereas through the ETC, 30 molecules of ATP can be produced for each molecule of oxidised glucose. The reason for this energy amplification ae well, comes from the ability to burn pyruvate and fatty acids within the ETC.
ATP ( Adenosine Triphosphate )
First of all why is ATP the energy ‘currency’ of all cells?. Because it is a molecular compound that provides the ability for multiple catabolic (break down) reactions to occur ( e.g ATP + 3 Phosphates = ADP, ATP + 2 Phosphates = AMP). Each reaction transfers energy for a particular function and then can be recycled by an anabolic (build up) process that reattaches inorganic phosphate (Pi) to create more ATP. In essence ATP lends itself to store energy and then be recycled again.
The high concentration of protons produced by the electron flow through the ETC, establishes an electrochemical proton gradient between the the inner mitochondrial membrane and the Matrix, and since the membrane is impermeable to protons, the protons accumulate there. Embedded in the membrane are enzyme complexes called ATP synthase in which protons can pass through a hydrophilic pathway of the enzyme complex across the inner mitochondrial membrane, allowing them to flow down the electrochemical gradient. As these ‘ions’ thread their way through the enzymatic process they are used to bind a phosphate group (Pi) to ADP (Adenosine Diphosphate ). ADP is constructed from Adenine a DNA/RNA nucleobase or purine base and when combined with a sugar molecule it becomes a nucleoside called Adenosine This can then chemically accept a phosphate group, in our case, 2 phosphate groups, where it becomes Adenosine Diphosphate or ADP. A process called phosphorylation occurs at complex V which involves ADP and the addition of another phosphate group thus synthesising ATP which is then transported from the mitochondria to the cellular cytoplasm (Cytosol) where the energy is used. Since the cell typically contains thousands of mitochondria in an active cell like muscle, the cellular cytoplasm accumulates a lot of ATP energy and can make more mitochondria and produce more energy as per the demand from the host. ADP is one of the most important and most abundant molecules in the body and is an ingredient in DNA structure as well as to allow muscle contraction and initiate healing during a blood vessel breach, but its primary role in our discussion, is to store and release energy. So, the body is always primed to move, to use up this accumulated energy and is primed to create more energy if needed by the host.
Brown adipose tissue
In the previous paragraph we asked the question, ‘Why have complex IV stage in the ETC at all, and we answered this by pointing out that it acts as a buffer to slow down electron transfer using oxygen as a transport to accomodate energy storage rather than released as heat. However if the host has an amount of brown adipose tissue than this type of fat adipose tissue is used to keep the host warm by generating heat. A sufficient amount of brown adipose tissue accumulates in the body of the host if they live in colder climates, so the cells decouple complex IV in order for the energy generated is in the form of heat when the host is subjected to cold.
ATP Recycling
As Lee Know points out in his excellent book ‘Mitochondria and the future of medicine’ the heart contains approximately 0.7grams of ATP, enough to contract the heart 60 beats/minute, but in one day the heart beats approx 86,400 times requiring 6,000 grams/day of ATP. Some ADP remains in the cytosol for ADP-ATP synthesis to provide energy to move ions across the membranes, but the rest must be recycled and sent back from the cytosol to the mitochondria to synthesis ATP again without the need to activate all of the preceding steps of the ETC which will take longer, and be unable to supply the massive energy requirements for the heart. There is one problem however, the mitochondrial inner membrane is impermeable to both ADP and ATP because of their high negative charge, so within the inner mitochondrial membrane there are embedded carrier proteins called ADP/ATP Translocase, and within the human eukaryotic cells there are 4 types SCL25A4, 5A5,5A6 and 5A31, that constitute more than 10% of the overall proteins embedded in the membrane. The Translocase is essentially a 6 alpha helices antiporter ( allowing reverse direction of substances ) that binds the 2 molecules of ADP/ATP transporting them from cytosol space into the mitochondrial matrix. This action however, is energetically expensive, using some 25 % of the energy produced from the ETC, but it is worth the expenditure just to maintain rapid ATP production to meet the needs of the host, ensuring constant ATP flow to the cytosol and ADP flow back to the matrix. I have to say the body is truly a remarkable intelligent organism.
Nutrient requirement for the ETC

So why have I gone through this lengthy explanation of mitochondrial energy production ?. Because it requires certain nutrients to drive this process and if you are nutrient deficient it might explain why you might be lethargic and tired and HAVE NO ENERGY. At this juncture let us reproduce the diagram ‘B vitamins and minerals involved in cellular energy production’ that first appeared in Part 2 from the article series 90 essential nutrients.
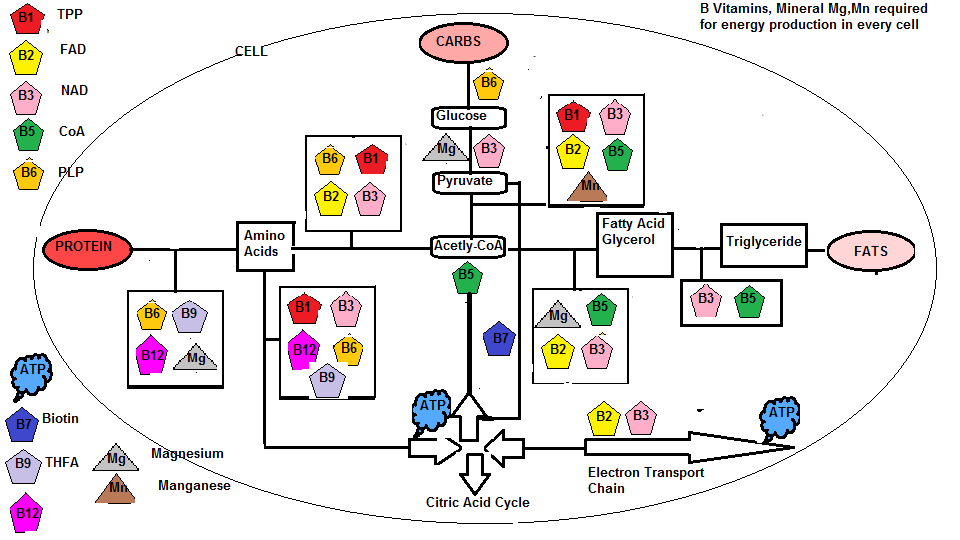
You will recall the Pyruvate Dehydrogenase complex which oxidises pyruvate to produce Acetyl-coA ( A process called pyruvate decarboxylation which delivers an acetyl group). However, acetyl-coA that feeds the TCA cycle ( citric acid cycle ) requires the metabolism of dietary food in the form of carbohydrates, protein and fats. From the diagram above it is crucial that an adequate supply of the B vitamin complex, Biotin, Magnesium and manganese is available. For instance Thiamine ( Vit B1), Riboflavin (Vit B2), Niacin (Vit B3), Pantothenic Acid (Vit B5), mineral Manganese, and amino acid cysteine are used for the conversion of pyruvate to Acetyl-coA. In fact, acetylated ( chemical introduction of an acetyl group) Pantothenic acid (B5) is the coA from Acetyl-coA. Glycolysis ( the conversion of glucose to pyruvate ) requires Niacin (B3) and Magnesium. The decarboxylation (a chemical reaction that removes a carboxyl group and releases carbon dioxide(CO2)) of Thiamine is produced from coenzyme Thiamine pyrophosphate (TPP). Minerals Magnesium is bound to ATP (MgATP) for muscle movement and Manganese as part of the Pyruvate Carboxylase enzyme. This enzyme speeds up the carboxylation of pyruvate to Oxaloacetate and the coenzyme involved which is N-Carboxyl Biotinyl lysine relies on Biotin (B7) for its synthesis This carboxylation of pyruvate to Oxaloacetateis an important anaplerotic reaction (reactions that produce metabolic pathway intermediates) that occurs in the TCA cycle, since it is responsible for replenishing the oxaloacetate ( formed by combined pyruvate molecules) increasing the TCA cycles capacity to metabolize acetyl-coA during increased energy needs.
The NAD (Nicotinamide Adenine Dinucleotide) molecule used in the TCA cycle, that is derived from amino acid tryptophan relies on Niacin (B3) for its synthesis, and FAD (Flavin Adenine Dinucleotide) relies on Riboflavin (B2) for its synthesis. From the above diagram Folic acid (B9) is required to synthesize coenzyme THFA (Tetrahydrofolic Acid) along with Pyridoxine (B6), Cobalamin (B12) and mineral Magnesium to break down dietary protein into the individual amino acids. In fact Niacin (B3) and Riboflavin(B2) are used to convert pyridoxine (B6) into its cofactor Pyridoxal Phosphate and Folic acid (B9) synthesis of folate metabolite 5-methyltetrahydrofolate into biochemical forms that the body can use. The ATP molecule is composed of Adenine, D-Ribose and 3 phosphate groups. D-Ribose is a 5 carbon sugar that is part of Riboflavin (Vitamin B2) found in foods like mushrooms, beef and poultry, cheddar and cream cheese, milk, eggs, caviar, anchovies, herring and sardines and Yoghurt/Kefir, almonds, spinach, asparagus, broccoli. There appears to be a massive push to sell D-Ribose supplements to promote longer exercise routines and boost energy, but if you are eating the correct food as listed above I don’t see the need to take these supplements and again I question, does the body process these supplements correctly whereas with food designed for the body is correctly utilized.
Nutrient sources
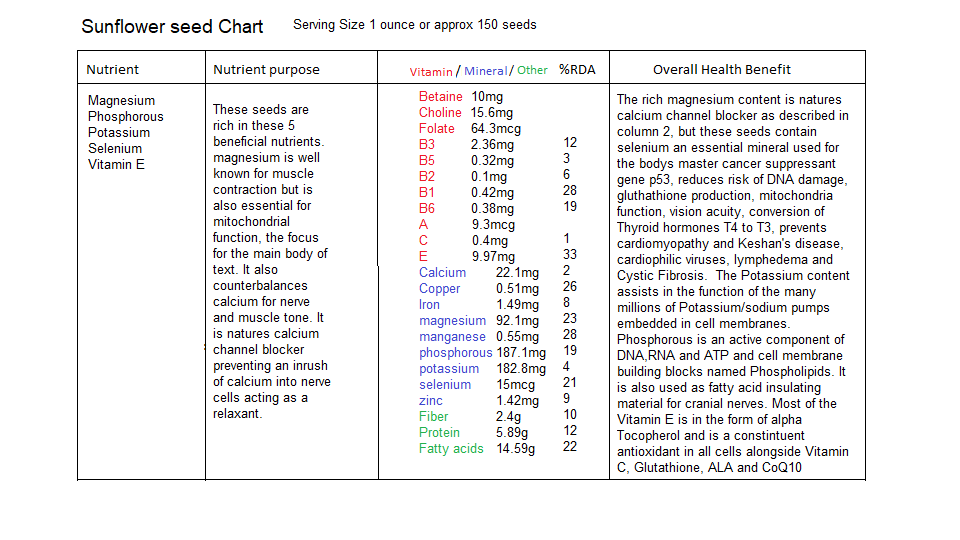
There are many food sources for the nutrients described above but I have included some seeds and grains that are rich in the required minerals Manganese and Magnesium as well as some B vitamins. The following are charts depicting what nutrients are contained in sunflower seeds, quinoa and oatmeal:
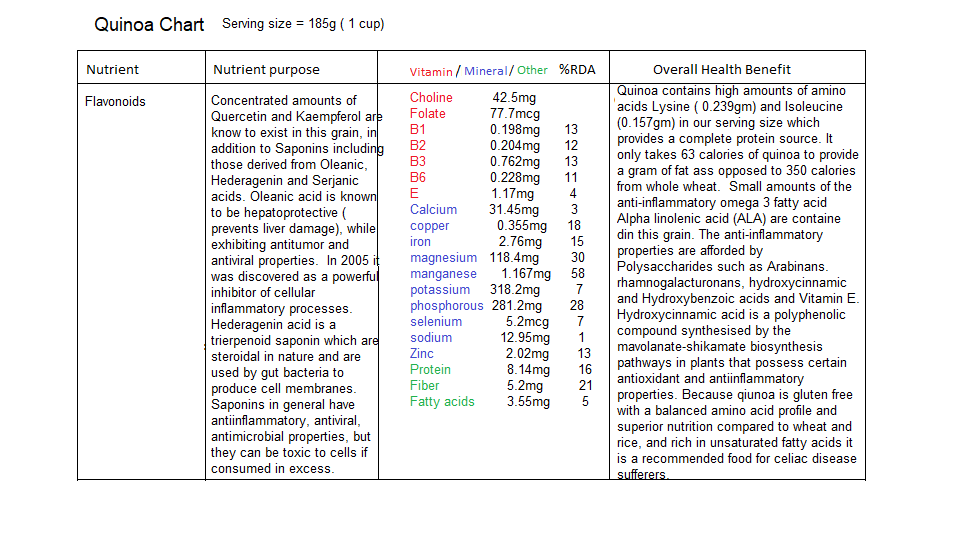
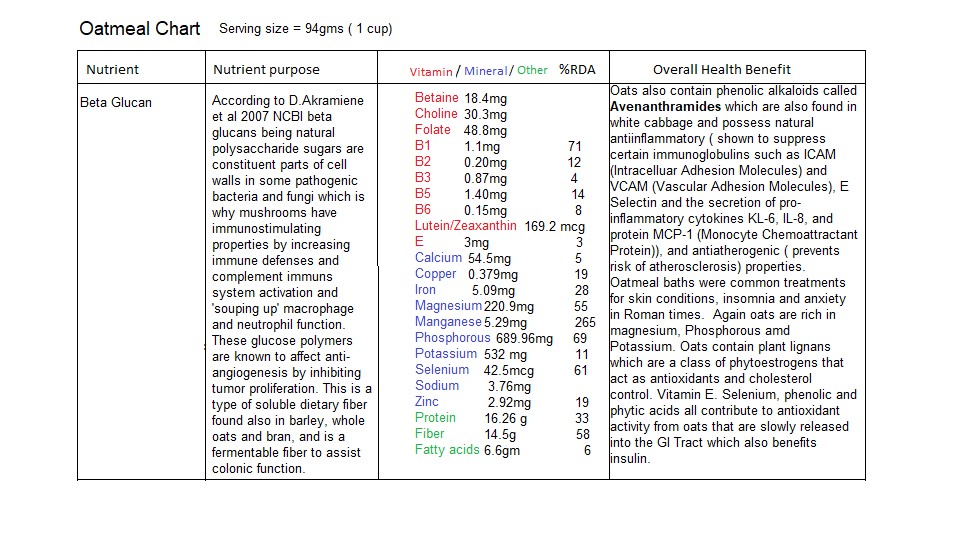 There is however one nutrient that is absolutely essential for efficient ETC function and that is Coenzyme Q10. I want to draw your attention to the mevalonate pathway that we discussed in the article ‘How cholesterol lowering drugs damage the body’ and its relationship to the ETC.
There is however one nutrient that is absolutely essential for efficient ETC function and that is Coenzyme Q10. I want to draw your attention to the mevalonate pathway that we discussed in the article ‘How cholesterol lowering drugs damage the body’ and its relationship to the ETC.
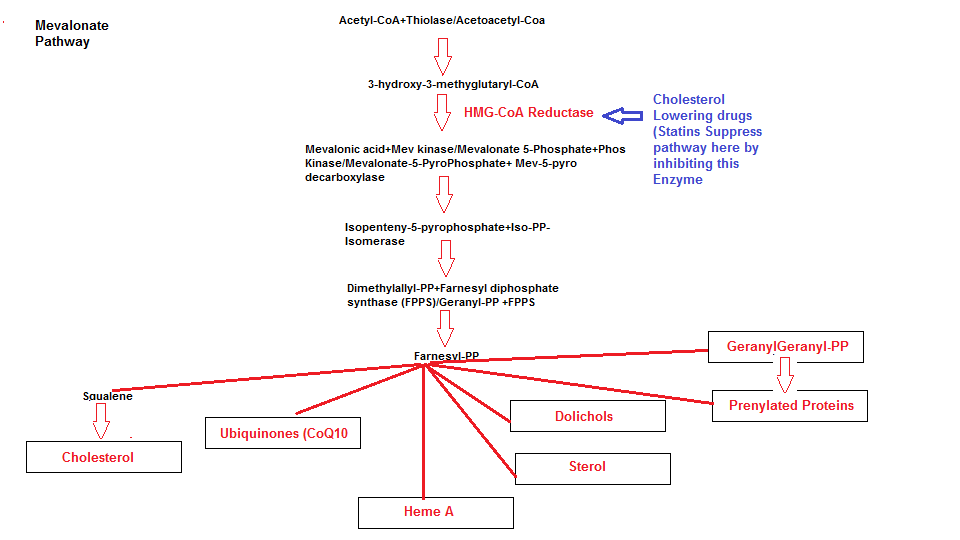
Not only do cholesterol lowering drugs slow or inhibit the manufacturing of cholesterol but they inhibit the production of other substances that are required for the ETC. For this concluding section I would like to draw upon certain text from previous articles. The following are products from the mevalonate pathway that are seriously impaired as a result of suppressing the main pathway enzyme HMG-coA Reductase.
Dolichols
Dolichols provide outer cell membrane integrity and since dolichols are part of the membrane structure they act as a form of lining. The dolichol acts as an ‘armor shield’ by preventing cell damage by providing the membrane with resilience, flexibility and permeability. Dolichols also acts as free radical scavengers in the cell membranes and Ciosek and colleagues observed a significant decrease in dolichol levels after Lovastatin ( a cholesterol lowering drug ) administration in vivo models causing oxidative stress and mitochondrial damage.
Heme A
If we take another look at the ETC:
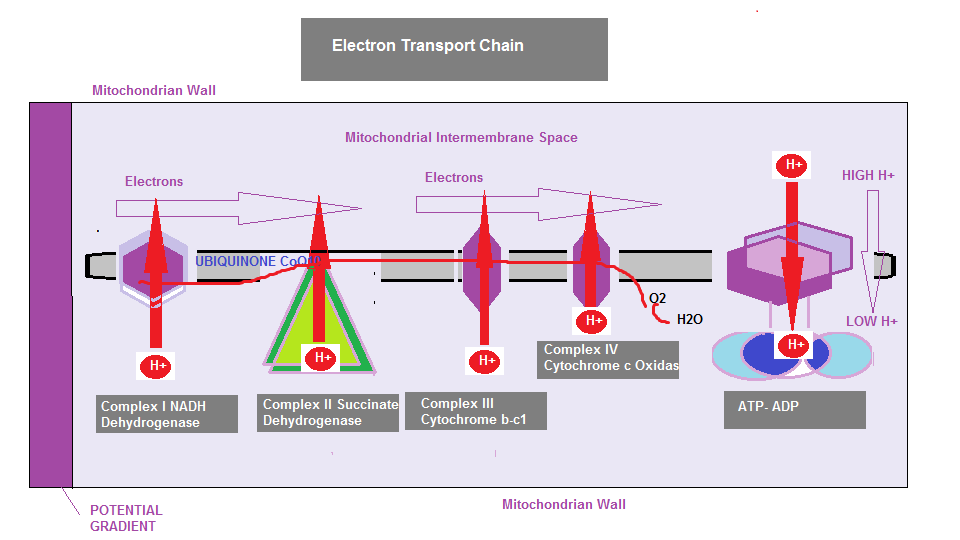
If you look at the Electron Transport Chain (ETC) diagram again you will notice that Complex III and IV are referred to as Cytochrome b-c1 and Cytochrome c oxidase. You will recall that energy for the cell is generated by electron transfer through the ETC by pumping protons from the mitochondria into the intermembrane space which then creates an electrochemical proton gradient across the membrane. Each of the complexes are embedded in the inner membrane, where 3 are proton pumps, electrically connected by lipid and water soluble electron carriers. Cytochromes are heme proteins (containing iron) that are primarily responsible for ATP generation. The cytochrome b-c1 is a monomeric (binding protein), and Cytochrome c oxidase is a larger enzymatic complex that catalyse redox (Oxidation-Reduction) reactions. If electron transfer is suppressed or Heme A is suppressed as is a consequence when statin drugs are introduced into the mevalonate pathway, complex III may begin to leak molecular oxygen resulting in superoxide formation. This was discovered in a 2015 study, affecting lung cancer cells The statin cholesterol lowering drugs are a class of drug that are described as `Amphiphilic` meaning, they contain in their chemical structure both Hydrophilic (Water soluble) and Lipophilic (fat soluble) properties, allowing them to breach the cell membrane to deliver the chemicals within the drug. However, this membrane breach degrades the lipids in the membrane so much so, the cell wall deteriorates, causing sodium to leak out, and as a consequence the cell will lose energy. Over time, the cell will become more permeable. Again,the problems all stem from mitochondrial dysfunction, specifically, interference within the the Electron transport chain. In the case of lung cells, they are vulnerable to oxidation damage, since they are responsible for taking in oxygen and transporting it to the bloodstream. Smokers are also vulnerable, since, under these conditions the lung capacity suffers from insufficient energy, and since the degraded cell wall becomes compromised, added to which a compromised antioxidant defense afforded by CoQ10 and the dolichols the ETC becomes dysfunctional from various angles. You can appreciate that suppression of multiple products CoQ10, Dolichols and Heme A can have potential disastrous effects on mitochondrial function.
Coenzyme Q10
Alternative medicine practitioner Dr Whitaker filed a petition in May 2002 to the FDA to mandate a warning be included in the package inserts of all statin drugs complete with a ‘ Black Box’ warning that surround the mandated required text:
Warning: HMG CoA reductase inhibitors (statin drugs) block the endogenous biosynthesis of an essential cofactor, coenzyme Q10, required for energy production. A deficiency of coenzyme Q10 is associated with impairment of myocardial function, with liver dysfunction and with myopathies (including cardiomyopathy and congestive heart failure). All patients taking HMG CoA reductase inhibitors should therefore be advised to take 100 to 200 mg per day of supplemental coenzyme Q10.
Alas his recommendation was ignored by the FDA.
Within the ETC it is necessary to convert Coenzyme Q10 ( the oxidised form known as Ubiquinone) into its reduced active form Ubiquinol which is no problem if you are younger than 30. However when in your 30’s and approaching 40 this conversion becomes inefficient slowing down the the functioning of the ETC, so it is recommended to supplement with Ubiquinol to bypass this conversion stage. I submit that one good reason for people that complain of chronic fatigue is due especially to the deleterious effects of these cholesterol lowering drug.
Conclusions
I hope this article explains clearly why the 90 essential nutrients are so called essential ,because they are the raw material that your body needs to run its various biological processes, and in this case cellular energy production. One last note, is to make you aware that generating new mitochondria occurs from exercise or extreme calorie restriction. Research in 2010 claimed that a compound found in various foods including CHOCOLATE, banana, kiwi, orange, papaya,apple,spinach, celery. Parsley, milk, eggs and other vegetables (Potatoes) and legumes contains PQQ (Pyrroloquinoline Quinone) which protects mitochondria from oxidative damage and stimulates the growth of new mitochondria, but I will leave you to do the research.
Lewis Thomas American Physician 1913-1993
Check out other Articles in this series:
Nutrients in Food and their bodily purpose I (Phenols)
Nutrients in Food and their bodily purpose II (Lignans, Triterpenes, Phytosterols, Carotenoids & Fats)
Nutrients in Food and their bodily purpose III (Phenolic acids, sulphur, sulphides,sulphoxides )
Nutrients in Food and their bodily purpose IV (Glucosinolates, Sulforaphane, Indole-3-Carbinol)
Nutrients in Food and their bodily purpose V (Lipid distribution, absorbed fats, Criciferous Veg)
Nutrients in Food and their bodily purpose VI (Nutrients required for Liver Detox)
Nutrients in Food and their bodily purpose VII (Seeds & the Omega Fatty Acids)
Nutrients in Food and their bodily purpose IX (Water I Properties and Body fluids)
Nutrients in Food and their bodily purpose X (Water II Cellular Hydration)
Nutrients in Food and their bodily purpose XI (Water III Fluid filtration, reabsorption, excretion)
Nutrients in Food and their bodily purpose XII (Water IV Blood pressure, Blood volume regulation)
Nutrients in Food and their bodily purpose XIII (Water V Body Fluid Dysfunction
Nutrients in Food and their bodily purpose XIV (Dental Nutrients)
Nutrients in Food and their bodily purpose XV (Nutrients involved in Methylation I)
Nutrients in Food and their bodily purpose XVI (Nutrients involved in Methylation II)
Nutrients in Food and their bodily purpose XVII (Nutrients involved in Methylation III)
Nutrients in Food and their bodily purpose XVIII (Nutrients involved in Methylation IV)
Nutrients in Food and their bodily purpose XIX (Methylation V and the Microbiota I)
Nutrients in Food and their bodily purpose XX (Methylation VI and the Microbiota II)
Nutrients in Food and their bodily purpose XXI (Superfoods: Wheatgrass)
Nutrients in Food and their bodily purpose XXII (Superfoods: Adaptogens)
Nutrients in Food and their bodily purpose XXIII (A look into our nutritional past Sir Robert McCarrison)
Nutrients in Food and their bodily purpose XXIV (Pregnancy: Nature vs Nurture vs Nutrition)
References/Acknowledgments :
- Sunflower seeds, Quinoa, Oatmeal nutritional value and analysis Nutritionvalue.org
- Avenanthramide, Salicylic acid, Beta-glucan, intercellular adhesion molecule, lignans, Nitric oxide synthase, Oleanolic acid, Triterpenoid saponins, Hederagenin, Sejanic acid, Rhamnogalacturonan, Hydroxybenzoic acid, Hydroxycinnamic acid, Mitochondrial Matrix, Cytochrome c, Cytosol, Glycolysis, Gluconeogenesis, Nicotinamide adenine Dinucleotide (NAD), Adenosine Diphosphate (ADP), Decarboxylation, Oxaloacetic acid, Anaplerotic reactions. Pyruvate carboxylase, ADP/ATP Translocase Wikipedia
- Arabinan and Arabinan rich pectic polysaccharides from quinoa Science direct
- Mitochondria diagrams google
- The role of Mitochondrial Porins and the permeability transition pore in learning and synaptic plasticity Edwin J Weeber et al 2002 Journal of Biological chemistry
- 90 essential Nutrients Part 2 article extremehealthacademy.com
- Electron transport chains and their proton pumps Molecular Biology of the cell 4th edition, Bruce Alberts, 2002 NCBI Bookshelf
- Fundamentals of human nutrition/Electron Transport chain Wikibooks
- Mitochondria and the future of medicine Lee Know ND 2018
- Mevalonate pathway blockade,Mitochondrial dysfunction and Autophagy a possible link Paulo Tricarico, Sergio Crovella, Fulvio Celsi 2015 International Journal of Molecular sciences
- How cholesterol drugs damage the body article extremehealthacademy.com
- PQQ Foods Michael Rucker
- Lewis Thomas AZQuotes
Author: Eric Malouin